Source Credit: CHINA DAILY
The World Health Organization (WHO) has donated 2,160 doses of an experimental Ebola vaccine to Uganda, marking a crucial step in combating the Sudan strain of the deadly virus. This initiative aims to evaluate the vaccine’s efficacy, particularly in light of a recent outbreak that claimed the life of a health worker in Kampala, Uganda’s capital.
Collaboration and Research Efforts
The clinical trial is being spearheaded by Uganda’s Ministry of Health in partnership with the Makerere University Lung Institute, the Ugandan Virus Research Institute, and a team of international experts specializing in filoviruses and vaccine trials. Regulatory authorities have also been involved to ensure a smooth and ethical research process.
According to a statement from WHO, the primary objective of the trial is to assess whether the vaccine can provide effective protection against the Sudan strain of Ebola virus disease (SVD). If proven effective, it could not only help end the ongoing outbreak but also serve as a preventive measure for future epidemics.
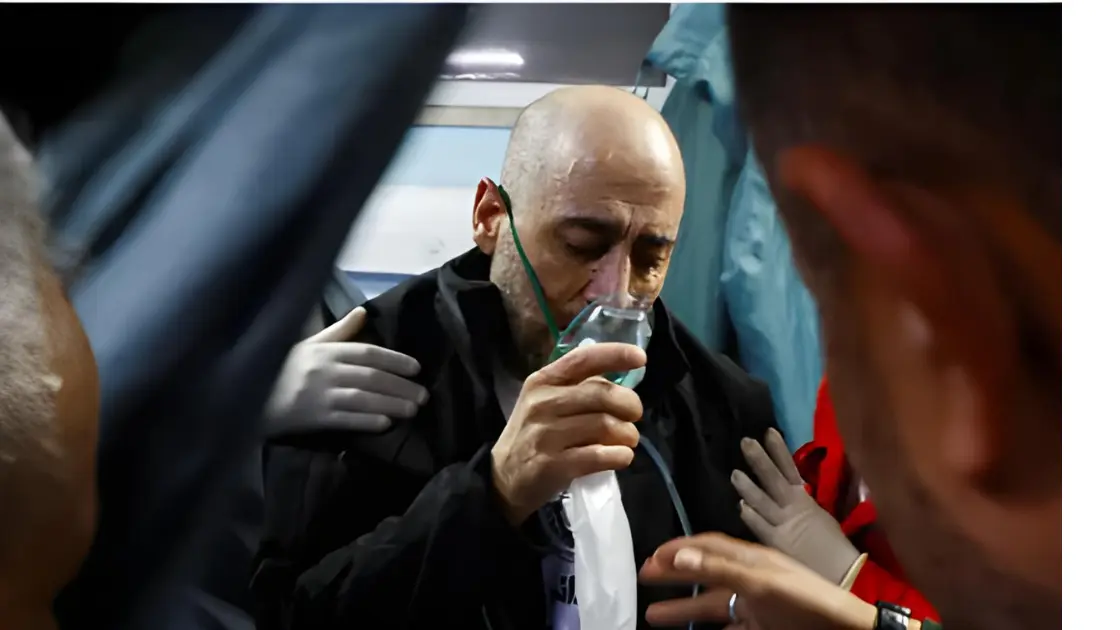
Recent Ebola Outbreak in Uganda
The Ugandan Ministry of Health declared an outbreak of Sudan Ebola virus on February 1, 2025, after the death of a 32-year-old male nurse at Mulago National Referral Hospital in Kampala. This marks the eighth outbreak of the Sudan Ebola virus in Uganda.
Unlike the Zaire Ebola virus strain, which has approved vaccines and treatments, there are currently no licensed vaccines or treatments for the Sudan strain. WHO emphasizes that early supportive treatment can significantly reduce fatalities, but the need for a specific vaccine remains urgent.
Who Will Be Vaccinated?
WHO has outlined that the vaccine trial will focus on individuals who are at the highest risk of contracting the disease. This includes:
- Close contacts of confirmed Sudan Ebola virus cases
- People exposed to individuals who have died from SVD
- Healthcare workers and frontline responders working in affected areas
The trial sites will be determined based on the locations where these high-risk individuals reside.
Previous Outbreak and Vaccine Development
Uganda’s last outbreak of Sudan Ebola virus occurred between September 2022 and January 2023, resulting in 164 confirmed cases and 77 deaths.
During that outbreak, WHO formed a committee of external experts to assess various candidate vaccines for potential trials in Uganda. The vaccines identified were then prepared for future use in case of another outbreak, leading to the current clinical trial process.
Progress of the Vaccine Trial
According to WHO, the trial preparation includes:
- Orientation of research teams on trial procedures
- Logistics arrangements for vaccine storage and distribution
- Deployment of research teams to affected regions to collaborate with surveillance teams
- Awaiting final regulatory approvals before full-scale implementation
WHO also highlighted that the case fatality rates of Sudan Ebola virus disease have varied between 41% and 100% in previous outbreaks, underscoring the urgency of effective medical interventions.
Global and Regional Implications
The success of this vaccine trial could have far-reaching implications for Uganda and other African nations vulnerable to Sudan Ebola outbreaks. By developing and testing a viable vaccine, scientists and health authorities hope to prevent future Ebola-related public health crises and enhance pandemic preparedness in the region.
As part of a broader effort to combat infectious diseases, WHO and Uganda’s health ministry encourage global cooperation, further research, and continued investment in vaccine development.
The trial’s findings will be closely monitored, and if successful, the vaccine could become a vital tool in protecting communities at risk of Sudan Ebola virus outbreaks.